
Lean Six Sigma is not quality
By Jennifer Dawson, MHA, LSSBB, CPHQ, DLM(ASCP)SLS, QIHC, QLC
Now before the entire industry lynches me for the sacrilege of the title of this article, let me explain what I mean. I have known Lean Six Sigma skeptics and outright haters and trust me, I am not one. I have completed graduate level Lean classes and Six Sigma black belt training on my own accord. I believe that the methodologies are sound, effective and definitely have their place. Allow me to explain my viewpoint. Let’s first evaluate what is meant by Lean and Six Sigma, because although they are often used together and sometimes interchangeably, they are not the same thing. According to the American Society for Quality (ASQ), the terms are defined as follows:
Lean manufacturing/production: An initiative focused on eliminating all waste in manufacturing processes. Principles of lean manufacturing include zero waiting time, zero inventory, scheduling (internal customer pull instead of push system), batch to flow (cut batch sizes), line balancing and cutting actual process times. The production systems are characterized by optimum automation, just-in-time supplier delivery disciplines, quick changeover times, high levels of quality and continuous improvement.
Six Sigma: A method that provides organizations tools to improve the capability of their business processes. This increase in performance and decrease in process variation lead to defect reduction and improvement in profits, employee morale, and quality of products or services. Six Sigma quality is a term generally used to indicate a process is well controlled.
Can a company achieve all of its quality goals by relying on Lean and Six Sigma alone? From my experience, the answer is no. Lean and Six Sigma will help the company to eliminate waste, reduce variation and continuously improve its processes. Six Sigma and lean manufacturing are business improvement methodologies, tools really, that should be viewed as part of a continually improving quality management system (i.e., ISO 9001, ISO 15189, CLSI QMS). Lean and Six Sigma is not a QMS or a replacement for a QMS. In order to understand what I mean here, it is helpful to review the ASQ definition of a QMS.
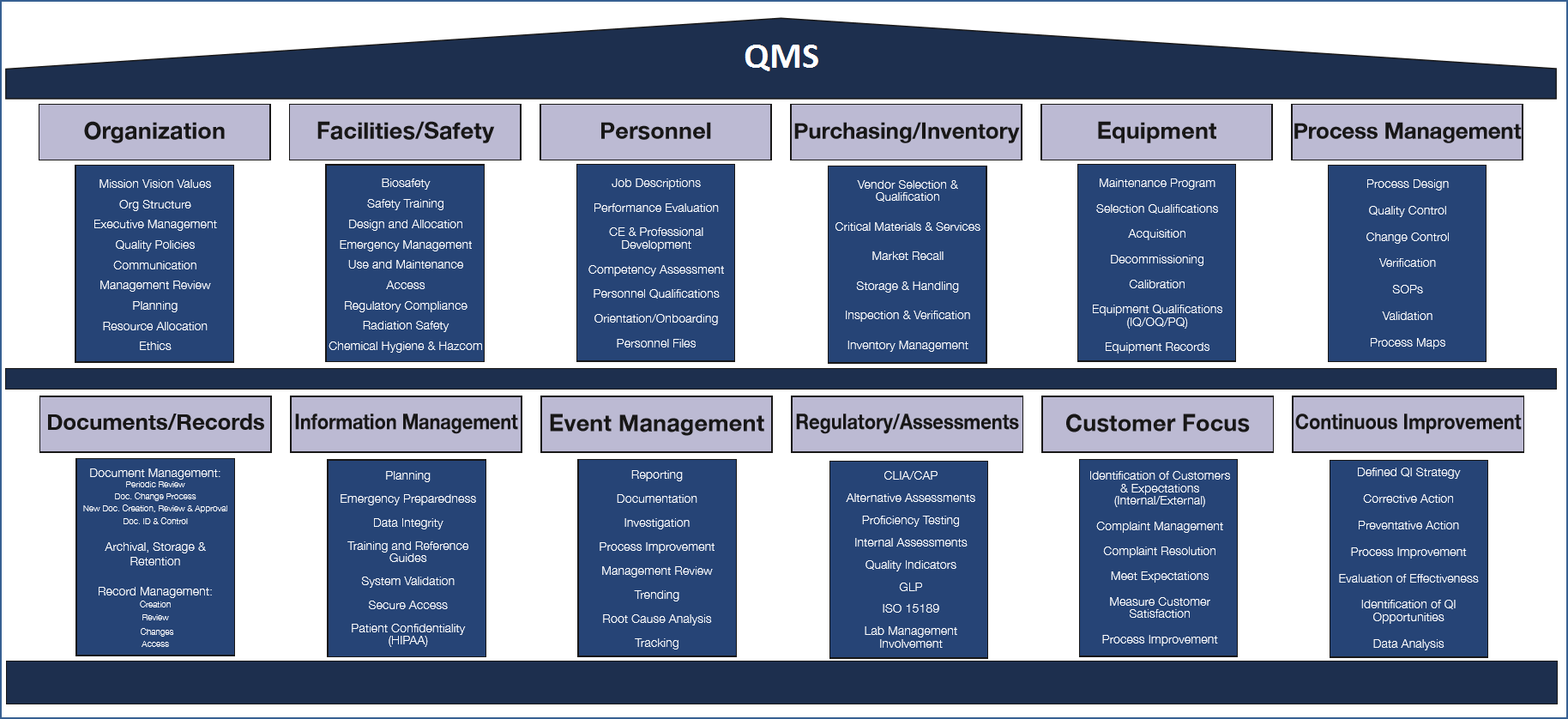
Quality management system (QMS): A formalized system that documents the structure, responsibilities and procedures required to achieve effective quality management, the act of overseeing all activities and tasks needed to maintain a desired level of excellence Lean and Six Sigma typically focus on eliminating waste, reducing variation and improving processes performed to make the service or product itself. Quality Management Systems are more holistic and focus on ensuring that policies, processes and procedures are in place to achieve the organization’s quality goals to meet customer expectations, regulatory requirements and to achieve business excellence. Lean and Six Sigma are continuous improvement tools that can be considered a subset of a QMS. Using the Clinical and Laboratory Standard Institute (CLSI) Quality Management System framework, detailed in QMS01 (refer to figure 1), you will see that process improvement tools such as Lean and Six Sigma fall under the pillar of continual improvement. ISO 15189’s section 4.12 is also focused on continuous improvement. Lean and Six Sigma, if you chose to employ them, should be fully integrated into your quality management system. It is certainly optional for a laboratory to implement Lean and/or Six Sigma. A Quality Management System, whether adopted from a standard or guideline or completely home grown, however, is imperative to a laboratory’s success. A QMS includes systems and documented policies that allow you to manage your organization to meet your quality goals. A QMS includes quality planning at an organizational level including resource allocation and goal setting. It includes a management review process where metrics are evaluated and results assessed. Other key components of a QMS are training and competency assessment, supplier selection and qualification, equipment maintenance, document control, change control, proficiency testing, employee health & safety, internal auditing, quality control and validation/verification. Non-conforming event and complaint management are also key components of a QMS. A standardized non-conforming event management process ensures that the organization is systematically identifying, triaging and correcting issues as they arise. Without documented systems for these fundamental business activities that contribute to quality, it is highly likely that things will fall through the cracks and patients and the laboratory will suffer. It is my personal view that the benefits of a Quality Management System are taken for granted and organizations look for the next new trendy quality initiative. The return on investment to be gained by investing further in a Quality Management System is overlooked. Instead leadership thinks that they’ve gained all there is to gain from a QMS and move on to the next initiative. This leaves the employees confused and feeling like they are subject to the newest quality “flavor of the month”. I find that it is often the inclination to put the basic framework of a QMS in place because it is the requirement of an accrediting body, but labs rarely build out and optimize the QMS to reap any real benefit beyond a piece of paper on the wall. As an example, I worked at a lab where they had a basket with some preprinted index cards to log complaints. It technically met the CAP checklist requirement, so it allowed the lab to pass the inspection, but when you evaluated the effectiveness of this complaint management approach, you found that no one had ever filled out one of the cards and if they had, there was no defined mechanism for review, trending or remediation. This system existed to meet an accreditation requirement, to check the box for the piece of paper on the wall, rather than to capture opportunities for improvement and improve the business. Contrast that to another lab where I worked, where we had a defined algorithm for what constituted a complaint and when to report, an electronic non-conforming management system with frequent trending and a standardized corrective action and effectiveness check process. The laboratory and its customers truly benefited from the latter approach while the former existed solely to gain accreditation. From my experience auditing labs throughout the country, this is often how Quality Management Systems are viewed. Just as QMSs are underrated, I believe that Lean and Six Sigma are overrated and represented as a complete solution for quality in clinical laboratories. Given the importance and necessity of Quality Management Systems in our highly regulated industry, when was the last time you saw a lab quality professional job advertisement that required or preferred experience with quality management systems such as CLSI, ISO 15189 or ISO 9001? I guarantee that if you have looked, you have seen many lab quality jobs requiring experience in lean and/or six sigma. If a lab doesn’t at least have a basic QMS effectively addressing fundamental elements for lab quality such as document control, non-conforming event management and management review, will they really be able to complete Kaizen events and green belt projects? Will they incorporate the Voice of Customer into their requirements when they haven’t thoughtfully trended or addressed the data from their CAP required customer satisfaction survey? Will they perform Failure Modes and Effects Analysis when they don’t consistently capture or remediate non-conforming events? The lack of awareness about what a QMS can do for an organization, in my opinion, is largely due to the difficulty in translating the benefits of a QMS in financial terms. Not much attention has been given to this topic in the lab industry. The idea of Cost of Poor Quality is gaining traction and is a way to demonstrate the benefits of a QMS to the financial decision makers. By quantitating and tracking the cost savings and cost avoidance achieved through remediation of rework and other non-conforming events, financial benefit can be demonstrated and attention of the C suite can be gained. In my mind, this is how Lean Six Sigma gained so much popularity so quickly as we are being asked to cut costs and do more with less. It was supported and championed by executive decision makers because of its easily captured financial, efficiency and quality benefits. Can your lab fulfill all of its obligatory regulatory requirements and ensure a high level of patient safety and employee health and safety using Lean and Six Sigma alone? Very unlikely. Are Lean and Six Sigma useful tools to employ as part of your lab’s quality management system? Absolutely. Let’s get the focus back on the big picture for quality in our labs. The bulk of our attention for lab quality should be in developing and maintaining comprehensive quality management systems. Lean and Six Sigma alone do not equate to lab quality.
Figure 1: Actual QMS Framework with site specific modifications that I have used in my labs (adapted from CLSI document QMS01, reference 2 below)
References
(1) American Society for Quality, www.asq.org, Accessed 8/6/2017
(2) CLSI. Quality Management System: A Model for Laboratory Services: approved Guideline - Fourth edition. CLSI document QMS01-A4. Wayne. PA: Clinical and Laboratory Standards Institute; 2011